The use or handling of health applications can entail a possible risk or hazard to the safety of patients either because of the functionalities they offer to the healthcare professional or because of the handling by the patient himself. To that goal, reasonable measures need to be taken to guarantee patient safety at all times and by all means. One of these measures is building in product quality.
Be aware that within the medical device world of quality, there is one rule to remember: if it is not written, it is not done.
Several standards will guide[1] the vendor reaching the sufficient product quality:
- IEC82304: Requirements for a Health Software product including Validation
- IEC62304: Software development life cycle processes (as part of IEC82304).
- ISO14971: Risk management for medical devices
- IEC62366: Usability engineering for medical devices
- ISO14155: Clinical Investigation
- ISO13485: Quality Management Systems (QMS) for medical devices including Validation
The next sections will detail out several elements of above standards.
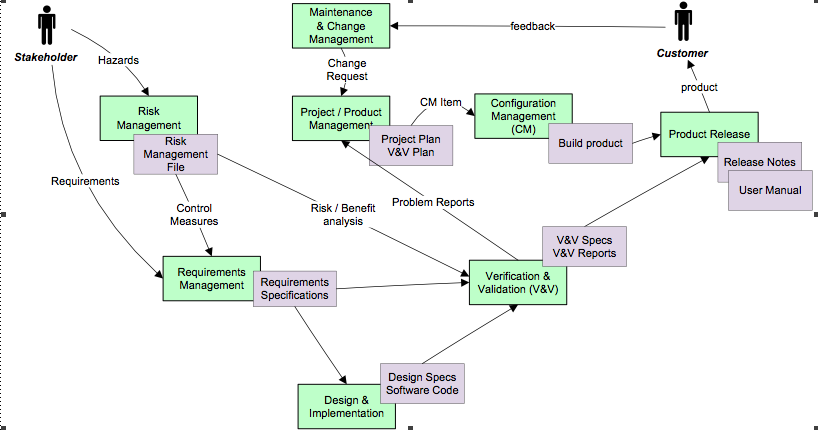
The picture below visualises the complete process
[1] If the software is a medical device (see section 8) than these standards are mandatory to follow.