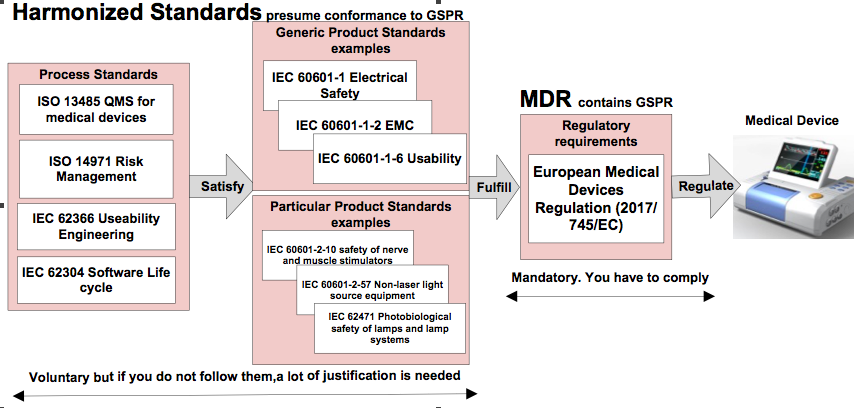
Harmonized standards and Common Specifications detail out the majority of the GSPR. If a medical device adheres to these standard, conformance to the GSPR is presumed. Common Specifications are still under development, harmonized standards are available for the predecessor of the MDR: The Medical Device Directive 93/42/EEC (MDD). Harmonized standards are divided in the following categories:
- Process standards defining how a medical device should be developed and maintained.
- Generic product standards defining product requirements for all medical devices.
- Particular product standards defining product requirements for specific medical devices.
The picture below visualizes above
The complete list of harmonized standard based on the MDD is published on the EU website:
As manufacturer, you have to pick the applicable particular standards for your product. In most cases the process standards and generic product standards are always applicable. The standards are input for the product requirements (see section Requirements Management) as regulatory requirements.